Charge & Current
Charge
Current
Conductivity
Materials have a property called conductivity which refers to how easily a current (flow of electrons) can pass through them.
Conductors: Easy Electron Flow
Insulators: Difficult Electron Flow
A majority of non-metals are not conductive i.e. are insulators.
Semiconductors: Controlled Conduction
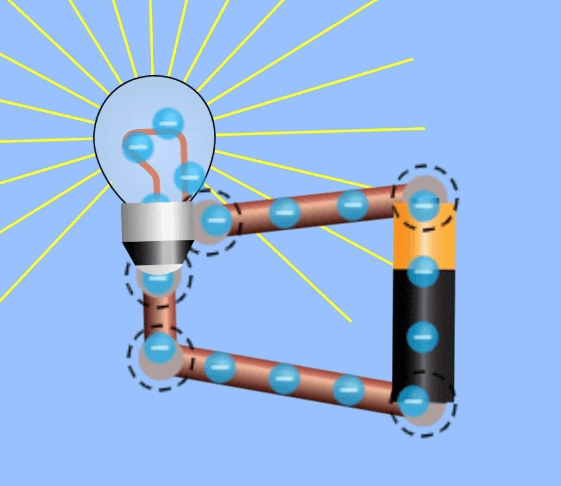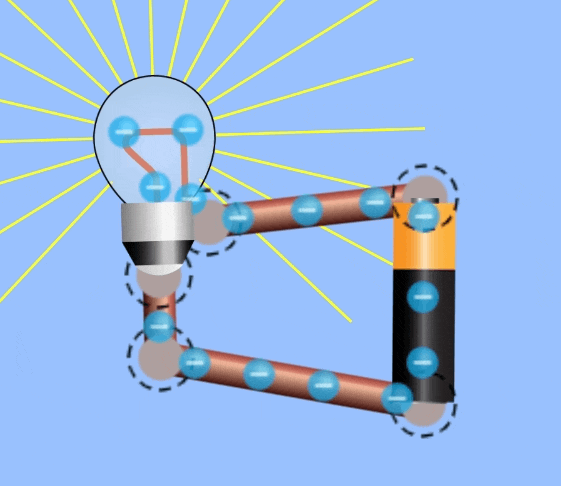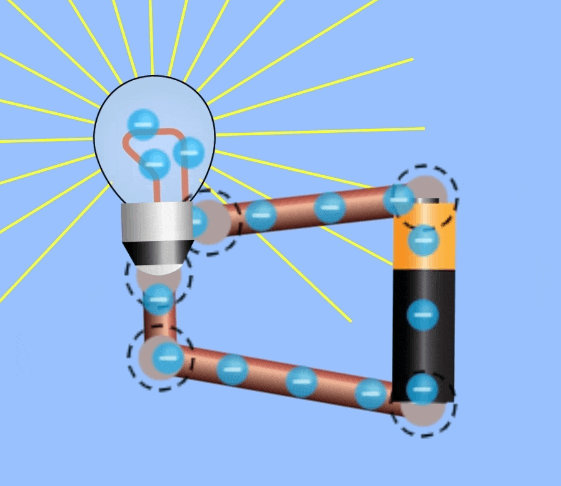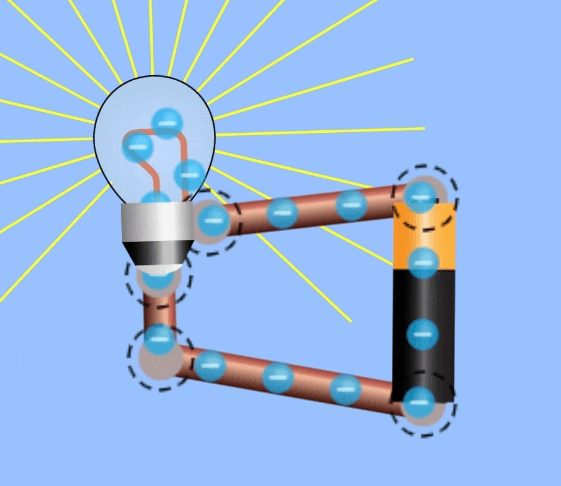
Electrical Circuits
In order to harness current to do work, an electrical circuit is required. An electrical circuit contains , in simplest terms:
- A closed loop of conductive materials, which is the path
- A power supply, consisting of many charged particles which can travel through the circuit.
- A load, to convert the electrical energy into another form, i.e. a lightbulb is a load that converts electricity to light energy.