Conductors are materials that easily allow charges to flow. Theses are usually metals, however a notable exception is graphite.
Properties:
When in Electrostatic Equilibrium, a conductor:
Most metals are good conductors due to their chemical structure. Due to having more electron shells, they have outer electrons which are weakly held, and can easily flow through to other metal atoms. This makes metals have a ‘sea of delocalised electrons’ amidst positively charged ions:
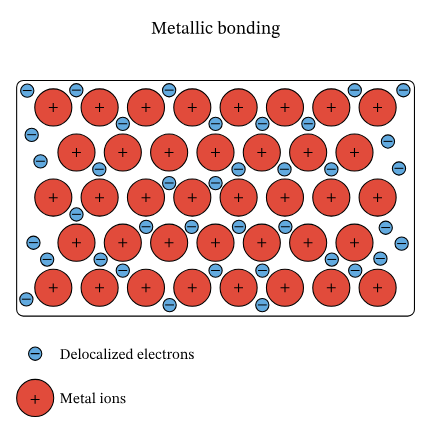
These free-moving negatively-charged electrons allow charges to easily be transferred throughout the metal, making it a good conductor.
Proof that electrons are the charge carriers was given by the Stewart-Tolman expirement
However, metals aren’t the only conductors. Graphite is the only non-metal conductor. This because it is essentially sheets of Carbon, stacked onto each other. The carbon atoms bonded in the sheets also have a free delocalised electron, which, just like in metals, can be used for charge transfer.
Examples
- Metals such as Copper (Cu), Silver (Ag) and Gold (Au)
- Some non-metals e.g. graphite.
- Liquids with dissolved salts
- Plasma (ionised gases) e.g. lightning, neon signs.